Background:
Options to treat elderly patients with newly diagnosed AML include intensive, attenuated chemotherapy, hypomethylating agents (HMA) and supportive care (SC). HMA have proven their efficacy in DACO-016 (NCT00260832) and AML-001 (NCT01074047) clinical trials, with a median overall survival (OS) of 7.7 months (95%CI, 6.2 to 9.2) with decitabine (DEC) vs. 5.0 months (95%CI, 4.3 to 6.3) with therapy choice (TC), considered SC or low-dose Ara-C (LDAC). Median OS was 10.4 months with azacitidine (AZA) (95%CI, 8.0 to 12.7) vs. 6.5 months (95%CI, 5.0 to 8.6) with conventional care regimens (CCR), considered standard induction chemotherapy, LDAC or SC. However, there are few direct comparative data of AZA and DEC in the context of trials or real-life settings.
Aims:
Here, we compared clinical outcomes between AZA and DEC in AML patients not eligible for intensive chemotherapy in the epidemiologic PETHEMA registry.
Methods:
We included newly diagnosed AML patients treated with AZA (75 mg/m2/d IV or SC days 1-7) or DEC (20 mg/m2/d IV days 1-5) that were not eligible for intensive chemotherapy.
Responses were recorded using IWG 2003 criteria. Rates of Complete Response (CR), complete response with incomplete recovery (CRi) and OS were co-primary endpoints.
Results:
Between 2006 and 2019, 638 patients were included. 497 (78%) received AZA and 141 (22%) received DEC as per physician judgement.
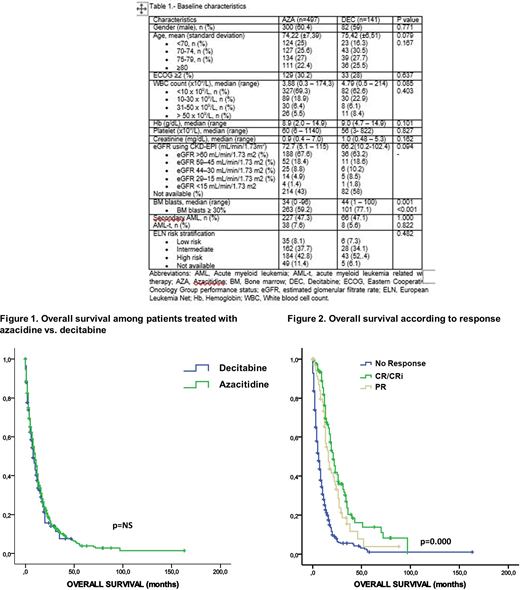
Baseline characteristics were comparable in both groups (Table 1), except for bone marrow blasts count ≥ 30%, which was more frequent in DEC group (59.2% vs 77.1%, p<0.001).
The CR rate was 16.3% vs 20.6% (p = 0.23); composite CR (CR+CRi) was 18.5% vs 22% (p = 0.35), and the overall response rate (ORR, partial remission (PR) plus CR+CRi) was 29.2% vs 34.8% (p=0.20); for AZA vs DEC, respectively.
A significantly higher ORR to AZA was associated with ECOG <2 (33.9% vs. 12.4% in patients with ECOG ≥2, OR 0.22, 95% CI 0.10 - 0.49, p=0.000), de novo AML (35.3% vs. 21.9% in secondary AML; OR 0.38, 95% CI 0.20 - 0.71, p=0.002) and estimated glomerular filtrate rate ≥ 45 mL/min/1.73m2 (30.4% vs. 9.3% in patients with estimated glomerular filtrate rate ≥ 45 mL/min/1.73m2; OR 0.15, 95% CI 0.034 - 0.67, p=0.013); while bone marrow blast count < 50% was the only factor influencing ORR to DEC (43.7% vs. 25% in ≥ 50% bone marrow blasts, p=0.029).
With a median follow up of 12 months, median OS was 10.0 (95% CI 8.7 - 11.2) vs 8.0 (5.7- 10.2) months for AZA vs DEC, respectively (p = 0.46) (Figure 1). Median OS was 21 (17.8 - 24.1) vs 16 (12.6 - 19.3) vs 6 months (5.0 - 7.0) for patients who achieved CR/CRi vs PR vs no response (p<0.001) (Figure 2).
Additional subgroup analyses by baseline characteristics performed to compare AZA vs DEC revealed that patients ≥ 80 years did benefit for treatment with AZA, median OS of 8 vs. 4 m (p=0.042), as well as patients with WBC ≥ 10 x109/L (8 vs. 5 m, p=0.036), platelet count <20 x109/L (8 vs. 4 m, p=0.021) and those with estimated glomerular filtrate rate ≥ 45 mL/min/1.73m2 (10 vs. 5m, p=0.033).
Conclusions:
This is a large retrospective comparison with long-term follow-up of clinical outcomes associated with AZA and DEC treatment for patients with AML patients not eligible for intensive chemotherapy. There were no significant differences in ORR, CR/CRi or OS between AZA and DEC. However, patients with WBC ≥ 10 x109/L, platelet count <20 x109/L and estimated glomerular filtrate rate ≥ 45 mL/min/1.73m2 could benefit from AZA in terms of OS.
Tormo:Janssen: Honoraria; Daiichi Sankyo: Honoraria; Servier: Honoraria; Roche: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria. Ramos:Amgen: Consultancy, Other: travel grant ; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: travel grant , Research Funding; Novartis: Consultancy, Other: travel grant; Takeda: Consultancy, Other: travel grant ; Daiichi-Sankyo: Other: travel grant ; Merck-Sahrp & Dohme: Other: travel grant; Rovi: Other: travel grant; Roche: Other: travel grant ; Jannsen: Other: travel grant; Abbvie: Consultancy, Other: travel grant .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal